test scripts for packaging equipment validation|packaging validation pillars : exporter exporters exporting Definition of Test Script - A test script is a document that contains a series of instructions to be performed to determine if the utility/system, equipment, or process functions as expected. It . Resultado da 11 de jun. de 2020 · Versões antigas do SnapTube. v20230715. Baixar. v20230608. Baixar. Snaptube 2023 v1. Baixar. Snap Tube 2022. Baixar. .
{plog:ftitle_list}
25 de jun. de 2023 · The free download of these parental control apps gives you limited features, but for advanced features, you can go for the premium version of FamiSafe. If you want an effective parental control, FamiSafe is the best parental control software to keep a check on your kid's smartphone. Best Parental Control Software - Android and iPhone - .
Definition of Test Script - A test script is a document that contains a series of instructions to be performed to determine if the utility/system, equipment, or process functions as expected. It .rigorous testing of packaging systems used with medical devices is mandatory in most major jurisdictions around the world. This UL white paper discusses the requirements and validation .
distribution testing and validation report. CONFIDENTIAL. Porous . Basic Packaging Validation Plan. Strength. Integrity; Microbial Barrier. Seal Peel; Visual Inspection; F1608. Burst Test: . use in your facility and/or with your equipment: Repeatability – variation within a lab. Reproducibility – variation from lab to lab .
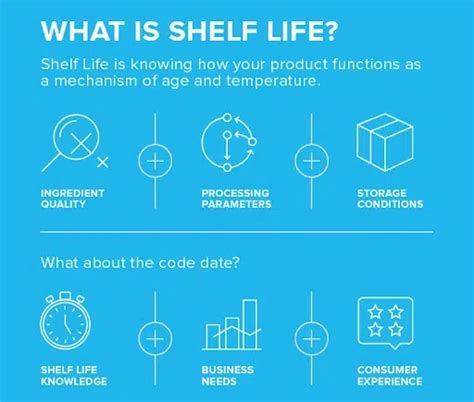
It may be necessary to test muliple packaging coniguraions if each presents a unique challenge to packaging stability over ime. How To Test? First identify how long is the tar get expiration date. Then, a study can be designed t o meet that timing. Keep in mind that real-time aging is a requirement. Execute the Validation Process Validation of packaging equipment is conducted through Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). Installation .Packaging Compliance Labs, a medical device packaging engineering, packaging validation lab, and contract packaging facility, has created an educational tool called “4 Pillars of Packaging Validation" to help guide packaging engineers through the process of meeting ISO 11607 expectations.
What Are Validation Protocol Test Scripts? Validation protocol test scripts provide a standard framework for the testing process. Each step in the testing process verifies specific pieces of the planning and specifications that were used to design the system. This is seen in the classic V model that is used in the GAMP guidelines. The left side .
Develop a Comprehensive Validation Plan: Before starting the equipment validation process, create a detailed validation plan that outlines the scope, objectives, and approach. Identify critical equipment parameters, acceptance criteria, and validation protocols. The plan should also include a timeline, the responsibilities of team members, and a risk .
The pharmaceutical and medical device industries adhere to various standards and guidelines to ensure the validation and testing of packaging materials and systems. These standards help maintain the quality, safety, and efficacy of products. Here are some key standards utilized for packaging validation and testing in these industries:Check out this comprehensive guide on Equipment Validation and discover expert strategies to ensure reliable equipment performance Product . CMMS Software; Work Order Software; . Test Scripts and Scenarios: Step-by-step instructions and scenarios for conducting specific tests during the PQ. These scripts provide guidance on executing the .
It also includes packaging validation items such as evaluation of equipment, protocol and report contents, amount of data (e.g. number of runs) and if warranted, microbiological studies. . Steps using packaging equipment should be evaluated to determine which steps or pieces of Any medical device package test that is used to support conformance with ANSI/AAMI/ISO 11607, Packaging for Terminally Sterilized Medical Devices—Part 1 and Part 2:2006, must be validated. While not a new concept, test method validation continues to challenge packaging professionals throughout the industry. Please don’t run vague test scripts because precise validation testing confirms systems perform as intended. Vendor Audit: Before purchasing bespoke or user-configurable software, a vendor audit should be performed to ensure that the software has been produced to appropriate quality standards.DDL – MN DDL, Inc. 10200 Valley View Road Eden Prairie, MN 55344 Phone: 952-941-9226 Fax: 952-941-9318
shelf life of packaging testing
Each category of testing has several sub-test methods. Medical device manufacturers refer to ISO 11607 to develop an appropriate test plan. Packaging Validation Testing Best Practices Guided by ISO 11607. ISO 11607 packaging for terminally sterilized medical devices is the principal standard for medical device packaging validation testing.
sample test scripts
Page 2 Guidance for Industry and FDA Staff General Principles of Software Validation In that case, the party with regulatory responsibility (i.e., the device manufacturer) needs to assess the Packaging Equipment Validation: A Comprehensive Guide Packaging equipment validation is a critical process in the pharmaceutical industry to ensure that the equipment meets regulatory requirements and consistently produces high-quality products. In this blog post, we will delve into the importance of testing scripts for packaging equipment . In the validation of people-dependent packaging processes, a key element is the elimination of controllable sources of variation. All equipment, materials, and components should be prequalified for the packaging operation. Where possible, the use of fixtures, holders, and special equipment should be implemented to reduce variation. The methods described above may be used, and the validation could also include altering the packaging configuration to test the system in a worst-case scenario. How to Vet a Testing Partner. While new packaging .
Updates to ISO 11607, Parts 1 and 2, have left many medical device manufacturers wondering about the future of their packaging designs. These changes come at a stressful time for OEMs, as Europe is also in the process of replacing the current Medical Device Directive (MDD) with the Medical Device Regulation (MDR) in 2020. The interplay between ISO 11607 and MDR is .
Examples are add-ons software for categories 3 and 4, MS Excel with VBA scripts, unique and dedicated systems, ERP systems, or developments of these facts to the specific needs of an organization, among others. In the validation strategy for this category of systems it is recommended to put more emphasis on: Specifications and testing modules Test Script Header. This section includes the Name, Title, and Run Number (if any) of the Test Script being executed. This section also includes a link to view the associated protocol document in a mini-browser window when the Test Script is not a stand-alone test script and is part of a Test Protocol. Navigation Panel
Best Practices for Writing Test Scripts. Crafting effective test scripts is a foundational skill for any software developer or tester, ensuring that applications perform as expected. To achieve this, it's essential to embrace certain practices that enhance the test script's clarity, maintainability, and effectiveness.
On the other hand, a Test Case is a step-by-step procedure used to test an application, while a Test Script provides instructions for automated application testing. There are three methods to create test scripts: record/playback, keyword/data-driven scripting, and writing code using a programming language.Responsible for validation services for a 140,000 sq. ft. cGMP contract packaging space for solid dose packaging of BFUs/CFUs and cards, as well as facility, utility and equipment qualification. As part of process validation through IQ/OQ/PQ, laboratories verify and validate that equipment performs to pre-determined specifications or criteria. ISO/IEC 17025:2017 Validation Requirements. ISO/IEC 17025 contains requirements for laboratories, including requirements for equipment used to make measurements within the laboratory. The .
Execute the Test Script: Run the automated test script against the application to verify its functionality. Analyze the test results for any issues or failures. Debug and Refine: If the test script encounters issues, debug the script and make necessary refinements to improve its accuracy and reliability.Leverage digital engineering tools, rigorous verification and validation processes, and automation tools to expedite the T&E planning, data analysis, reporting, and management of identified design shortfalls and . test data scoring boards to evaluate available test data for potential to meet any IOT&E and LFT&E requirements. 2.
otc 5606 compression tester
Characterize equipment operation processes and define critical process parameters and critical quality attributes; Write and execute the official test scripts, complete associated documentation for equipment and processes to ensure the test results are reported accurately. Job Types: Contract, Full-time. Pay: .00 - .00 per hour. Schedule:
otc 5606 compression tester kit
packaging validation pillars
Odds do Palpite: 1.78. Curtir o palpite (0) Faça uma aposta em Betano. Como fazer apostas? Aposta Ganha. O Athletic Bilbao vai receber o Barcelona na próxima quarta-feira (24), no gramado do Estádio San Mamés, em Bilbao, às 17h30 (horário de Brasília), em partida válida pela quartas de final da Copa do Rei da Espanha.
test scripts for packaging equipment validation|packaging validation pillars